Table Of Content
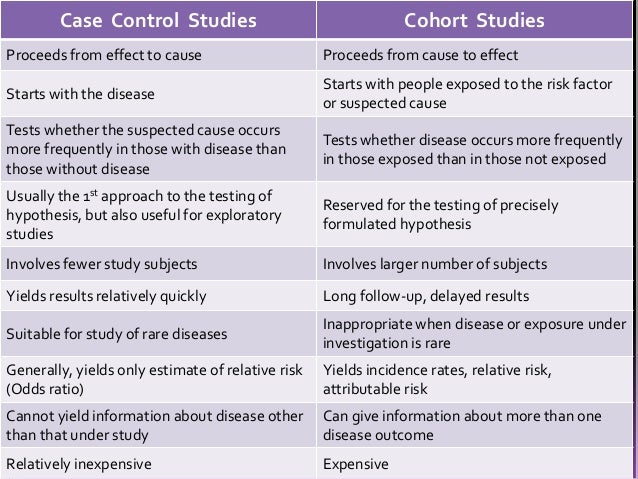
Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages. You would then collect information on any history of early life stress (e.g., abuse, neglect, trauma) for both the cases and controls and compare the two groups to determine if there is a relationship between early life stress and the risk of developing PTSD. You would then collect information on dietary intake of vitamin D for both the cases and controls and compare the two groups to determine if there is a relationship between vitamin D intake and the risk of developing osteoporosis. It’s important to remember that the case group is chosen because they already possess the attribute of interest.
Data Sources
The researcher might find that those with Kaposi's sarcoma are more likely to have HIV, and thus conclude that HIV may be a risk factor for the development of Kaposi's sarcoma. Case-control studies in India tend to be poor in quality because they are based on smallsample sizes. Small samples do not have sufficient statistical power to adjust for themultitude of confounding variables that bedevil research in psychiatry. Large samples cannotbe identified because India does not as yet have large electronic health care databases as asource of data. Case-control studies are prevalent in all fields of medicine from nursing and pharmacy to use in public health and surgical patients. Case-control studies are important for each member of the health care team to not only understand their common occurrence in research but because each part of the health care team has parts to contribute to such studies.
Case-control studies without matching
For example, Abdelmoneim and others29 specified a 120-day look-back period before the date of their cases (patients with acute coronary syndrome) to assess recent exposure to glyburide and gliclazide. Azoulay and others30 specified an exposure window of any time prior to a year before the date of cases in their study evaluating the association between pioglitazone and bladder cancer. If the investigators are collecting exposure data themselves, then outcome status should be blinded to study personnel. The case control study, while it has important strengths, is still a retrospective design with the problems that affect all retrospective studies. There may not be enough information available on enough subjects to analyze a particular factor of interest since the data collection was done after the fact.
Case–control study of the association of chronic acid suppression and social determinants of health with COVID-19 ... - Nature.com
Case–control study of the association of chronic acid suppression and social determinants of health with COVID-19 ....
Posted: Mon, 25 Oct 2021 07:00:00 GMT [source]
Case-control studies with matching
It is important to remember that the concordant pairs (pairs in which the case and control are either both exposed or both not exposed) tell us nothing about the risk of exposure separately for cases or controls. We may have to use sampling methods (such as random digit dialing or multistage sampling methods) to recruit controls from the population. A main advantage is that these controls are likely to satisfy the ‘study-base’ principle (described above) as suggested by Wacholder and colleagues. Furthermore, many of these controls will not be inclined to participate in the study; thus, the response rate may be very low. On the other hand, cross-sectional studies collect data on a population at a single point in time. The goal here is to describe the characteristics of the population, such as their age, gender identity, or health status, and understand the distribution and relationships of these characteristics.
GENERAL CONSIDERATIONS IN CONDUCTING A COHORT OR CASE–CONTROL STUDY
Like the cohort studies within which they are (at least conceptually) nested, case-control studies require an explicit definition of time zero, the time at which a choice is to be made between treatment strategies or protocols of interest [3]. Given a fixed cohort, time zero is generally determined by the defining event of the cohort (e.g., first diagnosis of a particular disease or having survived one year since diagnosis). However, while a fixed cohort may be ‘open’ to new members relative to calendar time, it is always ‘closed’ along the time axis on which all subject-specific time zeros are aligned. Cohort and case-control study designs are not “opposites” as are prospective vs.retrospective, or cross-sectional vs. longitudinal, or controlled vs. uncontrolled researchdesigns. Rather, like the randomized controlled and quasi-controlled designs, these designsare special kinds of research design in the controlled vs. uncontrolled classification. Notethat whereas a case-control study is always a special kind of controlled study, a cohortstudy can be classified under controlled or uncontrolled, depending on whether or not thereis a comparison group for the group of interest.
A Practical Overview of Case-Control Studies in Clinical Practice
Because of their efficiency, they may also be ideal for preliminary investigation of a suspected risk factor for a common condition; conclusions may be used to justify a more costly and time-consuming longitudinal study later. The weaknesses of case–control studies include inefficiency for studying rare exposures, difficulty of selecting unbiased controls, and inability to directly calculate incidence rates of outcomes. One of the major strengths of a cohort study is that the temporal sequence—drug exposure preceding outcome—is explicit in the study design. The incidence of a particular outcome among persons exposed to a certain drug can be directly calculated using a cohort design. Cohort studies are also relatively efficient for studying rare exposures, and multiple outcomes may be assessed for a single exposure.
WHY WE NEED COHORT AND CASE–CONTROL STUDIES
The factors (e.g., age, sex, time of hospitalisation) chosen to define how controls are to be similar to the cases are the ‘matching criteria’. The selected control group must be at similar risk of developing the outcome; it would not be appropriate to compare a group of controls who had traumatic corneal lacerations with cases who underwent elective intraocular surgery. In our example, controls could be defined as patients who underwent elective intraocular surgery during the same period of time.
As pharmacotherapy experts, pharmacists are continually updating their knowledge about drug effects. In addition to being knowledge users of research findings, pharmacists increasingly play a larger role in observational studies of drug effects. Observational studies are inherently nonexperimental and, unlike randomized clinical trials (RCTs), do not involve any manipulation (such as randomization) of the treatment and control groups by the investigator. The term ‘cohort’ may refer to either a ‘dynamic population’, or a ‘fixed cohort’, whose “membership is defined in a permanent fashion” and “determined by a single defining event and so becomes permanent” [9]. While it may sometimes be of interest to ask what would have happened with a dynamic cohort (e.g., the residents of a country) had it been subjected to one treatment protocol versus another, the results in this paper relate to fixed cohorts. Case-control studies are retrospective as researchers begin with an outcome and trace backward to investigate exposure; however, they differ from retrospective cohort studies.
Control group selection
An exciting feature for creators on the go is the integrated battery and Bluetooth connectivity. This makes the Micro Color Panel ideal for location shoots or editing suites with limited space. Even this small, it comes equipped with Bluetooth and a step tracker so that you can easily take control of your daily exercise.Effortlessly stylish, the GDB500 holds the key to all-new possibilities with G-SHOCK. Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Consider a situation in which a large number of cases of post-operative endophthalmitis have occurred in a few weeks.
Recall bias in a case-control study is the increased likelihood that those with the outcome will recall and report exposures compared to those without the outcome. In other words, even if both groups had exactly the same exposures, the participants in the cases group may report the exposure more often than the controls do. Recall bias may lead to concluding that there are associations between exposure and disease that do not, in fact, exist. A case-control study is a good tool for exploring risk factors for rare diseases or when other study types are not feasible. Many times an investigator will hypothesize a list of possible risk factors for a disease process and will then use a case-control study to see if there are any possible associations between the risk factors and the disease process. The investigator can then use the data from the case-control study to focus on a few of the most likely causative factors and develop additional hypotheses or questions.
If we sample the study participants based on exposure and move towards the outcome, it is a cohort study. However, if we sample the participants based on the outcome (some with outcome and some do not) and study the exposures in both these groups, it is a case-control study. For cohort studies, the drug–outcome association is usually expressed as a relative risk, a relative rate, or a hazard ratio. Advanced statistical techniques are used to account for factors other than the drug exposure of interest that might distort the drug–outcome association. These factors or potential confounders are often handled simultaneously with multivariable regression models. Because these statistical models account for measured variables, it is crucial that the data source capture as many potential confounding variables (or proxies of confounders) as possible.
In ‘The Dictionary of Epidemiology’ by Porta (2014), the authors have suggested that even though the term ‘retrospective’ was used for case-control studies, the study participants are often recruited prospectively. In fact, the study on risk factors for erysipelas (Pitché et al., 2015) was a prospective case case-control study. Thus, it is important to remember that the nature of the study (case-control or cohort) depends on the sampling method.
The potential for bias introduced by unmeasurable differences in patient selection, assessment, treatment, and follow-up is greater than in a rigorous prospective design. The immense power of prospective randomization to balance unknown and unmeasurable factors influencing outcome is not available to the retrospective researcher. Nonetheless, when a retrospective design is chosen for reasons of feasibility or practicality, it provides some of the strongest controls available under those constraints.
The investigator must put a great deal of effort into creating a proper control group to bolster the strength of the case-control study as well as enhance their ability to find true and valid potential correlations between exposures and disease states. The primary challenge in designing a case-control study is the appropriate selection of cases and controls. Poor selection can result in confounding, in which correlations that are unrelated to the exposure exist between case and control subjects. Confounding in turn affects estimates of the association between disease and exposure, causing selection bias, which distorts OR figures. To overcome selection bias, controls typically are selected from the same source population as that used for the selection of cases.
With these buttons at your fingertips, you can navigate your project timeline, set stills, and execute other commands with ease, significantly speeding up your workflow. Notably, some of these controls were previously exclusive to the larger DaVinci Resolve Mini and Advanced panels. Just in time for Apple’s May 7th event, Blackmagic announced the Micro Control Panel, a tiny, keyboard-sized controller that takes your iPad color grading to a whole new level.


No comments:
Post a Comment